Hydrofluorocarbon HFC-134a (R-134a), also known as 1,1,1,2-Tetrafluoroethane, is a commonly used compound in air conditioning and refrigeration systems. It’s a suitable replacement for older chlorinated compounds like R-12 and R-22, which are harmful to the ozone layer. HFC-134a is also used as an aerosol propellant and foam expansion agent due to federal regulations that mandate the use of compounds that are environmentally friendly.
Table of Contents
ToggleIndustry Uses
Refrigeration
1,1,1,2-Tetrafluoroethane (also known as HFC-134a) is an odorless, colorless, non-flammable gas that is commonly used as a refrigerant for various applications, including refrigeration and automobile air conditioning systems. This gas gained popularity in the early 1990s as a replacement for the more harmful refrigerant R-12, which was found to be contributing to the depletion of the ozone layer. Since then, 1,1,1,2-Tetrafluoroethane has become the preferred choice for its superior cooling properties and zero ozone-depleting potential. Retrofit kits are also available to convert older units that were initially designed for R-12, making the transition to this more environmentally friendly gas an easy and cost-effective process.
Aerosol
Norflurane (HFA-134a), also known as 1,1,1,2-tetrafluoroethane, is a chemical compound that finds its use as a propellant in most metered-dose inhalers used to treat respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD). It is considered entirely safe for this use, and there have been no reported instances of adverse health effects associated with its use. Although it is primarily used as a propellant, it has also been studied as a potential inhalational anesthetic. However, it has been found to be nonanesthetic at the doses used in inhalers, and hence, it is exclusively used as a propellant in medical applications.
Propellant
1,1,1,2-Tetrafluoroethane is a versatile gas that has found various uses in different industries. It is commonly used as a refrigerant in plumbing pipe freeze kits and has also been employed to cool computers in some overclocking attempts, like in the case of crypto coin mining. Additionally, it is a popular propellant for airsoft airguns, often used in place of CO2. The gas can also be mixed with a silicone-based lubricant and utilized as a propellant in building insulation foams, where it works alongside R-245 to create a highly effective insulation material. Overall, 1,1,1,2-Tetrafluoroethane has proven to be a valuable and versatile gas with a wide range of applications.
Solvent
1,1,1,2-Tetrafluoroethane is a colorless, odorless, and non-flammable gas that is commonly used as a refrigerant. However, it also has other applications, such as being an organic solvent that is suitable for extracting flavor and fragrance compounds. This is particularly useful as an alternative to other organic solvents, such as supercritical carbon dioxide. In organic chemistry, it can also be used as a solvent in liquid sub- and supercritical fluid forms, which makes it a versatile compound. Its unique properties make it an effective solvent that can be used in various industries.
Fire extinguisher agent
1,1,1,2-Tetrafluoroethane is a chemical compound that can be used as a substitute for sulfur hexafluoride, which is commonly used as a dielectric gas. While it may not be as effective in putting out electric arcs, it has properties that make it suitable for use in electrical insulation. In addition, it has been found to be a useful alternative to sulfur hexafluoride in the smelting of magnesium, where it is employed as a shielding gas.
Other uses
1,1,1,2-Tetrafluoroethane is truly remarkable in its versatility and range of uses. One of its most common uses is as a propellant for pharmaceuticals, particularly bronchodilators. It is also commonly used as a wine cork remover, in gas dusters (also known as “canned air”), and as an air dryer for removing moisture from compressed air. Additionally, it is used in plastic foam blowing and as a cleaning solvent. However, its usefulness continues beyond that. This substance is also a key component in particle detectors, such as the resistive plate chamber particle detectors utilized in the Large Hadron Collider. It is even used in cryogenic particle detectors. The diverse range of applications for R-134a is truly impressive.
Summary of applications
1,1,1,2-Tetrafluoroethane is widely used in the industry for the following applications:
- Cleaning products for removal of grease, flux, and other soils from electrical equipment;
- Refrigerant flushes;
- Products for sensitivity testing of smoke detectors;
- Lubricants and freeze sprays for electrical equipment or electronics;
- Sprays for aircraft maintenance;
- Sprays containing corrosion-preventive compounds used in the maintenance of aircraft,
- Electrical equipment or electronics, or military equipment;
- Pesticides for use near electrical wires, in aircraft, in total release insecticide foggers, or in certified organic use pesticides for which EPA has specifically disallowed all other lower-GWP propellants;
- Mold release agents and mold cleaners;
- Lubricants and cleaners for spinnerettes for synthetic fabrics; duster sprays specifically for the removal of dust from photographic negatives, semiconductor chips, specimens under electron microscopes, and energized electrical equipment;
- Adhesives and sealants in large canisters;
- Document preservation sprays;
- FDA-approved MDIs for medical purposes;
- Wound care sprays;
- Topical coolant sprays for pain relief;
- Products for removing bandage adhesives from skin;
- Military marine vessels where reasonable efforts have been made to ascertain that other alternatives are not technically feasible due to performance or safety requirements.
- Human-rated spacecraft and related support equipment where reasonable efforts have been made to ascertain that other alternatives are not technically feasible due to performance or safety requirements;
- Processing of botanicals for extracting flavoring and aromatic compounds.
Dosing
The U.S. Navy has requested that the NRC review the toxicity data on HFC-134a and recommend appropriate safety levels for emergency and continuous exposure. According to available information, no toxicity data is available on humans following exposure to HFC-134a. However, animal studies suggest that HFC-134a has low systemic toxicity levels following acute and chronic exposures. Neurotoxicity and cardiac sensitization may occur after acute exposures to HFC-134a at very high concentrations. The NRC will also identify research gaps that should be looked into to provide further information on the safety of HFC-134a.
Bans
In 2012, automobile manufacturers began transitioning to new, eco-friendly refrigerants to reduce the impact on the environment. By the year 2024, newly manufactured light-duty vehicles in the United States will no longer use HFC-134a in their MVAC systems, as per a July 2015 rulemaking. Instead, the HFO refrigerant R-1234yf will replace R-134a used in car air conditioning systems for newly manufactured medium-duty passenger vehicles, heavy-duty pickup trucks, and complete HD vans.
Recently, the US EPA has announced plans to ban several common refrigerants, such as R-134a, R-410A, and R-407C, for use in new chillers in the USA starting January 1, 2024. These changes will also impose future restrictions on using higher GWP HFC gases in new domestic and commercial refrigeration equipment, as well as prohibit the use of class 3 flammable refrigerants as retrofits. It’s worth noting that these bans are only applicable to light vehicle consumer refrigeration applications and will not affect the commercial use of R-134a.
Plant processing
When it comes to extracting compounds such as wax and acid from a substance, there are various methods available. Butane is a commonly used solvent for this purpose, and the standard post-processing protocol involves purging the dissolved butane using a vacuum oven for several days.
However, R-134a is a better alternative as it is much less miscible with the wax and acid compounds, making it easier to extract the desired compounds. The vendor’s process includes a built-in purge of R-134a, which takes around half an hour to an hour and reduces the concentration of the solvent to deficient levels.
Safety
It’s interesting to note that there have been rumors in the industry that R-134a may decompose to hydrogen and carbonyl fluoride at high temperatures, which could be harmful if inhaled. However, a 2014 study conducted at Perdue found that R-134a did not decompose to HF until temperatures exceeded 600 degrees Celsius, much higher than the temperatures at which hash oil is typically consumed.
According to the study, direct contact of 1,1,1,2-tetrafluoroethane with open flames or hot surfaces over 370 °C (698 °F) may cause vapor decomposition and the emission of toxic gases, including hydrogen fluoride and carbonyl fluoride. The concentrations of R-134a, HF, and CF in the case of R-134a testing are shown in Figs. 14, 15, and 16.
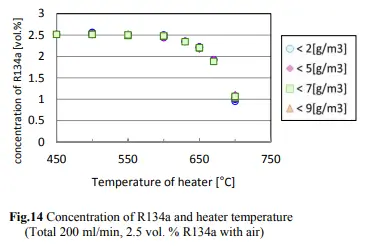
At heater temperatures between 500-550°C, a decrease in the concentration of R-134a and an increase in HF concentration was observed. Moreover, when the temperature exceeded 650°C, the generation of CF was noticed. Interestingly, the decrease in the R-134a concentration remained unaffected by the humidity levels.
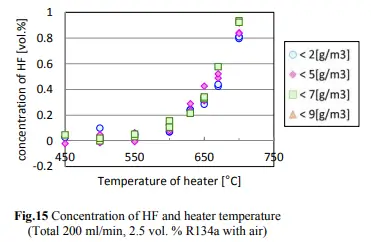
The conversion of R-134a to HF initiates at a temperature of 600C with a rate of 0.01% or 100ppm. However, the dabbing temperature of a pin is 300 degrees Celsius below the conversion temperature, which means that there will be no conversion of R-134a to HF occurring at that temperature. Nonetheless, if we assume that 600C temperature is achieved in some way, and if the allowed concentration for R-134a in an extract is below 20ppm, then the maximum HF conversion will be 0.2ppm (400ppb).
According to the National Institutes of Health (NIH) data, exposure to HF levels higher than 3 ppm for more than an hour can cause irritation. However, the maximum anticipated conversion will be 15 times below that limit. Besides, the dabbing temperature is significantly lower than the conversion temperature, making it unlikely to cause irritation.
The inhalation of hydrogen fluoride can cause critical respiratory tract irritation and induce respiratory disease. Animal models and controlled human exposure studies have documented respiratory tract irritation. Therefore, longer-term exposures or systemic effects require considering the total fluoride intake from all exposure routes, such as inhalation, dermal, and ingestion.
In summary, sensory irritation can occur at exposures greater than 3 ppm for one hour. Chronic exposure, which is repeated exposure lasting more than approximately 10% of the lifespan in humans or around 90 days to 2 years, necessitates evaluation of total fluoride intake from all exposure routes to assess longer-term exposures or systemic effects.
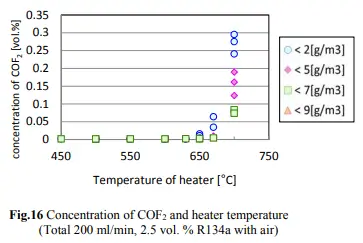
The conversion of R-134a to CF starts at over 650C with a rate of 0.02% or 200ppm. Keeping in mind that a butane flame burner temperature is over 300 degrees C while dabbing, there will be no conversion of R-134a to HF occurring at that temperature. However, if we assume that 650 degrees C is achieved (350 degrees C over the dabbing temperature) and if the allowed concentration for R-134a in an extract is below 20ppm, we are talking about max HF conversion of 0.4ppm (400ppb) in vapors.
According to the NIH, the irritation from CF occurs within the recommended exposure limit of 10 hours, with a time-weighted average of 2 ppm (5 mg/cu m). The maximum anticipated conversion will be five times below that limit, except that a pin’s dabbing temperature is 300 degrees C below the conversion temperature.
This technique has been employed and made famous by PURE5™ Extraction starting from the early 2000s. It is a superior choice compared to the other mentioned methods because it combines their benefits and removes their drawbacks. Because of its low boiling point, it operates similarly to CO2 and can be easily extracted and solvent fully recovered. This aerosol is a type of nonflammable gas that is a fluorinated hydrocarbon. It enables the processing of much bigger volumes and delivers more safely and cost-effectively.
In contrast to other techniques, this method does not extract wax and lipids and does not require winterization or extraction at extremely low temperatures. The process is secure, quick, and economically beneficial. At ambient temperature, R-134a aerosol can draw out live resin and full-spectrum extracts, along with their valuable medicinal properties, at an affordable price without compromising quality. Because of the increased energy efficiency and faster processing, the expenses related to the system and operation of the R-134a aerosol botanical extraction process are significantly reduced.
Benefits
Maintaining high Terpene content
Terpenes are highly fragrant chemicals that give plants and herbs their distinct scents. They are a class of natural products consisting of compounds with the formula. They have been used in perfumes, medicines, and flavorings since ancient times. Terpenes are natural compounds synthesized from isoprene units, which can be five carbon atoms connected to 8 hydrogen atoms configured in strains or rings. The terpenes define the type of strain genotype and its medicinal behavior.
Maintaining high antioxidant activity
Antioxidants are substances that can assist your body in defending against damaging free radicals associated with health issues such as diabetes and cancer. The plants contain a high concentration of flavonoids, specifically anthocyanins. Anthocyanins are a type of flavonoid compound. They can appear in various parts of higher plants, such as leaves, stems, roots, flowers, and fruits. They do not have a smell and mildly tighten the skin. An astringent, also known as adstringent, is a substance that causes the body tissues to contract.
Maintaining high Bioavailability
Various methods have been available for extracting botanical oils, and each method produces a different outcome. Maintaining the oil’s natural consistency and a neutral pH, which prevents oxidation, is essential. It is also crucial to preserve low-temperature compounds like enzymes, phenolics, and flavonoids and avoid the loss of active components such as terpenes and cannabinoids during extraction.
Mitigating Mold and Yeast
The evidence gathered indicates that using the R-134a aerosol significantly reduces the presence of mold and yeast by more than 1000 times compared to the initial contaminated substance. We offer an additional organic and pure method to restore the polluted plant by transforming it into a specific strain of terpene-rich Hash Resin™ extract.
Preserving Natural Color
To ensure that the extraction is strain-specific, it is necessary to thoroughly comprehend the flower, the solvent, and the extraction method employed. The purest method of extracting plants involves using fully developed strains and extracting them at ambient temperature with our highly inert solvent. That way, we ensure that the original components are not changed while maintaining the natural color and presence of high-quality compounds, keeping the extract natural.
Generic Solvent Properties
The extraction process’s effectiveness relies on the solvent distinctive selectivity. Each solvent has distinct characteristics in the process due to its ability to dilute different organic compounds selectively. This is why each extraction technique generates specific extract types, and solventless methods, predominantly involving mechanical separation, have lower selectivity and purity levels. The post-processing stage requires information about the extraction process since it varies depending on the type of crude extract obtained.
In the diagram provided, we present a compilation of typical solvents utilized in extraction processes along with the prevalent components they dilute from the plant. Acknowledging that botanical oils are the most abundant active compounds is crucial. When extracting those components, selecting the most suitable solvents in the field is necessary.
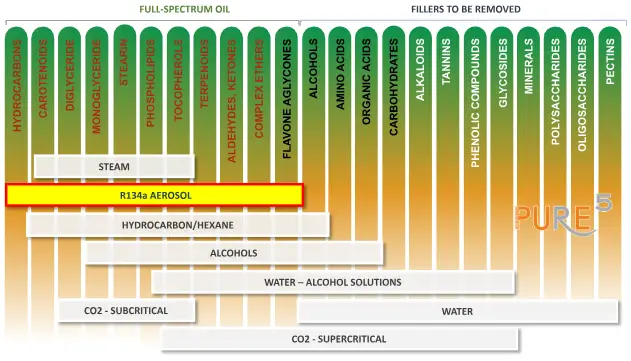
The extract from the plant can be used directly in a product containing only Full-Spectrum compounds, including the terpenes, or it may need to undergo winterization and additional filtration depending on the extraction method. Furthermore, it may be necessary to reduce the levels of THC in the CBD market to meet regulatory requirements. Once the waxes have been eliminated, the extract can undergo distillation to be transformed into isolates employed in pharmaceutical preparations.
Conclusion
The use of R-134a as a botanical processing solvent has been approved by the EU community since 1994 and FDA since 2001. As we outlined above, all the safety issues of utilizing the R-134a gas are entirely safe for human use and is the recommended replacement for the existing non-efficient CO2 and hydrocarbon extraction methods while providing many more benefits to the end product, resulting in natural extracts, pesticide, and mold suppression while preserving the organic properties of the extracts and the natural colors without extraction of undesired byproducts.
***
George Stantchev, PhD








