Hydrofluorocarbon HFC-134a (R-134a) or also known as 1,1,1,2-tetrafluoroethane is a gaseous halocarbon that is considered as a prime candidate for replacing other chlorinated halocarbon materials, such as Freon 12 and Freon 22, for use in air conditioning and refrigeration systems and as an aerosol propellant or foam expansion agent. This change is to a federal regulation that mandates switching from CFCs to other suitable compounds that do not damage the ozone layer.
Table of Contents
ToggleThe real question is: if HFC-134a is utilized to extract cannabis and there is a residual gas in the form of a liquid in the extract what are the potential safety concerns? For human consumption in the form of ingestion there exists an FDA exemption for flavors and flavorings for foods which states that a maximum of 1000ppm of residual can be present for human consumption. Albeit alternate industry universe the same should apply to cannabis edibles. As a function of design our extraction platform has been characterized with the maximum residual solvent of approximately 800ppm therefore below the established threshold value of 1000ppm.
GRAS EXEMPTION CLAIM
INEOS Fluor Ltd. hereby claim that the use of 1,1,1,2-tetrafluoroethane( HFC-134a) as an extraction solvent in the production of flavors and flavorings for foods is exempt from the premarket approval requirements of the Federal Food, Drug and Cosmetic Act because we have determined that such use of HFC-134a is generally recognized as safe (GRAS). Maximum specification residue in the food flavor extract is 1000 ppm
For inhalation via smoking the above does not apply. All extracts for smoking processed utilizing HFC-134a will go through a degassing process as part of the SOP and within 24 hours residual solvent is potentially removed to a non-detect state. Below you will read that most vape pens, dab rigs or nails will operate in the range 230 to 300 degrees C (450 to 570 F) to vaporize the smoke for inhalation. Remember combustion of cannabis (THC) occurs at 230C (450F). It has been a rumor in the industry that at very high temperatures 550 to 600C (1022f to 1202f) R-134a decomposes to hydrogen fluoride (HF) and carbonyl fluoride (CF) which may cause irritation when inhaled. We are going to review this topic based on scientific data of the actual effects. In general, direct contact of 1,1,1,2-tetrafluoroethane with open flames or hot surfaces more than 370 °C (698 °F) may cause vapor decomposition and the emission of toxic gasses including hydrogen fluoride and carbonyl fluoride. You can see the temperature in which these by-products are formed HF and CF are in a significantly higher range than the typical smoker. If you were to overheat your delivery device and these compounds are generated what is the effects?
According to NIH data the irritation from HF occurs at levels higher than 3ppm with exposure for over 1 hour. if we assume that 600C is achieved (which is 300 degrees C over the actual dabbing temperature) in some way and if the allowed concentration for R134a in an extract is below 20ppm we are talking about max HF conversion of 0.4ppm (400ppb). The max anticipated conversion will be 15 times below the limit of 3ppm. Last we must consider the fact that the dabbing temperature of a nail is 300 degrees C (572f) or less below the conversion temperature. The critical effects of inhalation exposure to hydrogen fluoride creating respiratory tract irritation and the induction of respiratory disease are documented in animal models and has been observed in controlled human exposure studies.
We stated earlier that all residual presence of HFC-134a can safely be removed without degradation of the extract. If a processor did not follow proper SOP and a 20ppm detection level was found, you can see this has smaller chances if any effects on humans. Let’s not forget that California residual solvent testing for Butane is up to 500ppm allowable levels you make up your mind. For detailed analysis please read the long version of the article.
Industry Uses
Refrigeration
1,1,1,2-Tetrafluoroethane is a non-flammable gas used primarily as a “high-temperature” refrigerant for domestic refrigeration and automobile air conditioners. These devices began using 1,1,1,2-tetrafluoroethane in the early 1990s as a replacement for the more environmentally harmful R-12. Retrofit kits are available to convert units that were originally R-12-equipped.
Aerosol
For its medical uses, 1,1,1,2-tetrafluoroethane has the generic name norflurane. It is used as propellant for some metered dose inhalers. It is considered safe for this use. In combination with pentafluoropropane, it is used as a topical vapocoolant spray for numbing boils before curettage. It has also been studied as a potential inhalational anesthetic, but it is non anesthetic at doses used in inhalers.
Propellant
1,1,1,2-Tetrafluoroethane has also been used to cool computers in some overclocking attempts as an example in crypto coin mining. It is the refrigerant used in plumbing pipe freeze kits. It is also commonly used as a propellant for airsoft airguns in substitution of CO2. The gas is often mixed with a silicone-based lubricant and also used as propellant in building insulation foams along with R-245.
Solvent
1,1,1,2-Tetrafluoroethane is also being considered as an organic solvent suitable for extraction of flavor and fragrance compounds, as a possible alternative to other organic solvents and more specifically the supercritical carbon dioxide. It can also be used as a solvent in organic chemistry, both in liquid and supercritical fluid form.
Cannabis processing
R134a is much less miscible with the extracts than butane is. Standard protocol for post-processing with butane is several days in a vacuum oven to purge the dissolved butane. There is a half-hour to an hour purge of the R134a built into the vendor’s process, which reduces the concentration to very low levels.
It has been a rumor in the industry that in very high temperatures R-134a decomposes to hydrogen (HF) and carbonyl fluoride (CF) which may cause irritation when inhaled. We are going to be reviewing this topic based on scientific data of the actual effects. In general direct contact of 1,1,1,2-tetrafluoroethane with open flames or hot surfaces in excess of 370 °C (698 °F) may cause vapor decomposition and the emission of toxic gasses including hydrogen fluoride and carbonyl fluoride.
A 2014 study done at Perdue found that there was essentially no decomposition of R134a to HF until the temperature exceeded 550 degrees C, which is much hotter than the temperatures that hash oil is typically consumed. The concentrations of R134a, HF, and CF in the case of R134a testing are shown in Figs. 14, 15, and 16.
The decrease in the R134a concentration and the increase in the HF concentration began when the heater temperature was in the range of 500–550°C, and generation of CF began when the temperature exceeded 650°C. The decrease in the R134a concentration was not affected by the humidity.
As one can see the conversion of R134a to HF starts at 600C with the rate of 0.01% or 100ppm. Having in mind that a butane flame temperature is 300 degrees C while dabbing there will be no conversion of R134a to HF occurring at that temperature. Although if we assume that 600C is achieved (which is 300 degrees C over the actual dabbing temperature) in some way and if the allowed concentration for R134a in an extract is below 20ppm we are talking about max HF conversion of 0.2ppm (400ppb).
According to NIH data the irritation from HF occurs at levels higher than 3ppm with exposure for over 1 hour. The max anticipated conversion will be 15 times below the limit beside the fact that the dabbing temperature of a pin is 300 degrees C below the conversion temperature. The critical effects of inhalation exposure to hydrogen fluoride are respiratory tract irritation and the induction of respiratory disease. Respiratory tract irritation is documented in animal models and has been observed in controlled human exposure studies.
According to the above, the sensory irritation can occur at exposures greater than 3 ppm for 1 h (Lund et al. 1997). Prolonged respiratory tract effects can occur after short-term exposure. To evaluate longer-term exposures or systemic effects, the total fluoride intake from all exposure routes (inhalation, dermal, and ingestion) must be considered (EPA 1988; NRC 2006). Chronic exposure is a repeated exposure lasting more than approximately 10% of the lifespan in humans or approximately 90 days to 2 years.
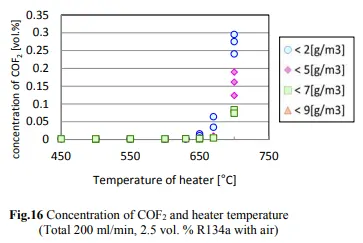
As one can see the conversion of R134a to CF starts at 650C with the rate of 0.02% or 200ppm. Having in mind that a butane flame temperature is 300 degrees C while dabbing there will be no conversion of R134a to HF occurring at that temperature. Although if we assume that 650C is achieved (which is 350 degrees C over the actual dabbing temperature) in some way and if the allowed concentration for R134a in an extract is below 20ppm we are talking about max HF conversion of 0.4ppm (400ppb).
According to NIH data the irritation from CF occurs in the recommended Exposure Limit is 10 Hr Time-Weighted Avg 2 ppm (5 mg/cu m). The max anticipated conversion will be 5 times below the limit beside the fact that the dabbing temperature of a pin is 300 degrees C below the conversion temperature.
Fire extinguisher agent
1,1,1,2-Tetrafluoroethane is also being considered as an alternative to sulfur hexafluoride as a dielectric gas. Its arc-quenching properties are poor, but its dielectric properties are fairly good. It has been used as an alternative to sulfur hexafluoride in magnesium smelting as a shielding gas.
Other uses
Other common uses include a propellant for the delivery of pharmaceuticals (e.g. bronchodilators), wine cork removers, gas dusters (“canned air”), and in air dryers for removing the moisture from compressed air, plastic foam blowing, as a cleaning solvent, etc. It is used in the resistive plate chamber particle detectors in the Large Hadron Collider. It is also used for other types of particle detectors, e.g. some cryogenic particle detectors.
Dosing
The U.S. Navy requested that the NRC review the toxicity data on HFC-134a and recommend 1-hr and 24-hr EEGLs. The Navy also requested that the NRC recommend a 90-day CEGL for HFC-134a and identify appropriate research to fill data gaps. We have collected the available supporting documentation and above evaluation in support of the dosing.
In general no toxicity data are available on humans following exposure to HFC-134a. Animal studies indicate that HFC-134a has a low level of systemic toxicity following acute, subacute, subchronic, and chronic exposures. For example, neurotoxicity and cardiac sensitization occur after acute exposures to HFC-134a at very high concentrations.
R134a Bans
In 2012, automobile manufacturers began the transition to new, climate-friendly alternative refrigerants. As a result of a July 2015 rulemaking, by model year 2024, the MVAC systems in newly manufactured light-duty vehicles in the United States will no longer use HFC-134a. The HFO refrigerant R1234yf, designed as a replacement for R134a in car air conditioning systems, is extended for use in newly manufactured medium-duty passenger vehicles, heavy-duty pickup trucks, and complete HD vans.
There was an indent of US EPA to ban a number of common refrigerants, including R134a, R410A and R407C, will be banned from use in new chillers in the USA from January 1, 2024. The wide ranging changes also place future restrictions on the use of the higher GWP HFC gasses in new domestic and commercial refrigeration equipment and ban the use of class 3 flammable refrigerants as retrofits. All those bans are applied in the light vehicle consumer refrigeration applications and will not be affecting the commercial use of the R134a.
References
Tetrafluoroethane – R134A
NBK231519 – R134A