Passing state regulations for microbial contamination is challenging if the crop has not been grown clean. High levels of mold, yeast, and bacteria can cause health problems for consumers when burned or extracted improperly. What happens when your flower fails your state’s compliance test? Do you have post-harvest treatment options that can save your buds? The good news is that the microbial contamination can be remediated without damaging the final product.
Table of Contents
ToggleThe tips that are usually given in such cases are to handle the material properly. This is considered storage and handling before extraction. Like keeping moisture away, use air tight containers, irradiate the flower after harvest to kill the unwanted microorganisms. Unwanted microorganisms not only degrade the material, but also secrete toxins. This way the damage can extend further if left unnoticed at the beginning. Although you have more options than that.
The most important factors are environmental conditions during the grow, harvest, drying, curing, repackaging storage, and the microenvironmental conditions of the packaged product across the supply chain from cultivator to consumer. Although there is one more big factor – the cannabis extraction which very oftenly is ignored. That factor can play a major role into the mold and yeast mitigation, we will discuss in details and examples below.
Controlling Microbial Growth
Water activity is used in many cases as a critical control point for Hazard Analysis and Critical Control Points (HACCP) programs. Samples of the food product are periodically taken from the production area and tested to ensure water activity values are within a specified range for food quality and safety. Measurements can be made in as little as five minutes, and are made regularly in most major food production facilities.
Water activity values can also help limit moisture migration within a food product made with different ingredients. If flower material is not cured to a water activity measure of at least 0.70, yeast and mold can grow on it. California has established an upper limit for the water activity for flowers of 0.65. How do you achieve the desired water activity of ≤0.65 post-harvest if, and when, your state mandates this test?
To achieve the desired water activity in the cannabis of 0.65, you will need to have a stable humidity across your entire set of post-harvest processes in this range of 55–65% RH. This stable relative humidity is called equilibrium relative humidity (ERH). If the relative humidity is not stable and controlled, you will not be assured of the desired water activity when the cannabis is packaged. To accomplish the ERH, you will need to monitor and control the potential effects on relative humidity from the weather, temperature, and people entering and exiting your processing rooms-to name just a few factors.
Once packaged, the microenvironment in the package can be controlled by including relative humidity control packets. This will be the ERH in the packaging that translates into the desired water activity measure that will prevent further microbial growth. The packets contain proprietary materials that can both absorb and release water into the packaging based on the relative humidity.
Killing Methods
There are few effective methods to reduce total mold and yeast count in post-harvest cannabis, but the two below seem to have minimal impact on the quality of the flower (terpenes, cannabinoids, and appearance). They are effective treatments and are not cost-prohibitive. The decontamination machines may be leased or purchased from PURE5TM Extraction.
X-Ray Chamber
X-ray chamber decontamination is highly effective and has a history of use in medicine although x-ray is harmful for all living beings if overexposed and that is the reason for consideration in botanical remediation. X-rays are a form of high-energy electromagnetic radiation. X-ray wavelengths are much shorter than those of ultraviolet (UV), visual light (VL) and thermal infrared (LWIR) radiation. This means they pack more energy with higher levels of intensity and therefore are much more effective in killing microbes than UV. The instrumentation for X-ray decontamination is well-tested and uses technology that has been proven for decades.
Simply the cannabis is placed in a lead-lined chamber that ensures the safety of the operator during the decontamination process. X-rays are produced from an internal vacuum tube once the lead lined chamber is sealed. The X-rays penetrate the cannabis and kill the microbes. The system does not allow the operator to be exposed to X-rays but safety precautions should be taken using the machine. It is highly effective in the destruction of the full complement of microorganisms. After the processing by X-ray chamber decontamination, your cannabis will be ready to pass the state tests. Although keep in mind that the x-rays are beams of high energy electrons and it is possible to alter the cannabinoids and terpenes by slightly oxidizing them.
Ozone Chamber
Once placed in the chamber and sealed in, the cannabis will be exposed to ozone flow, and the microbes on the surface will be killed. Any ozone not consumed by the disinfection process is deactivated by the system before the door can be opened. This method greatly reduces the number of microbes. Ozone chamber decontamination has been demonstrated to be effective against the full range of microorganisms. As a side effect it may also cause some of the cannabinoids to oxidize.
Such safe and easy to use ozone chambers are built and offered by PURE5TM Extraction for flower and extract remediation while utilizing the seed to shelf PURE5TM Extraction process the remediation reactors are universal equipment that mitigates any remaining mold and yeast before the extraction process.
Mitigating Mold with Extraction
Every extractor knows that to have good extract on the output you need good input material. Unfortunately, this is not always the case, beside a poor potency material very often there are other factors such as mold and microbial contaminations. Below we will discuss another option to mitigate molds in your end product with minimum steps and keeping the extracts with no byproducts created from the remediation.
Solvent based extraction is another way to handle mold and yeast products. The most important thing to understand is the selectivity of the solvent and what solvent will mitigate the mold because not every solvent can do it. Extracting cannabis, the target active ingredients are the oil soluble essential oils and oleoresins (cannabinoids) where the molds are water soluble compounds that are easy to be separated with the correct methods.
Each extraction method delivers quite different extract consistency. That is so because of the dielectric properties of the solvent usually addressed as polar and nonpolar molecules which defines the extraction selectivity. It is essential to understand the nature of the active ingredient to be obtained. In the case of extracting similar compounds, extract similar compounds so the non polar oils will be extracted with non polar solvents.
The molds are non polar carbon based compounds that are mostly acidic in appearance and are easy to be washed out with water based or water miscible solvents. That is why ethanol and CO2 methods are not mitigating the mold during the extraction and even make it worse by multiplying its appearance in the final extract.
Why Extraction is important?
Below is the chart showing all organic compounds contained in the plant and all major solvents used to extract them. One can see that each solvent pulls different active compound consistency and that is the reason for different extract quality and post processing required to purify the extracts. The plant compound can be separated in 3 groups depending on their affinity to the solvents: oil soluble, alcohol soluble and water soluble.
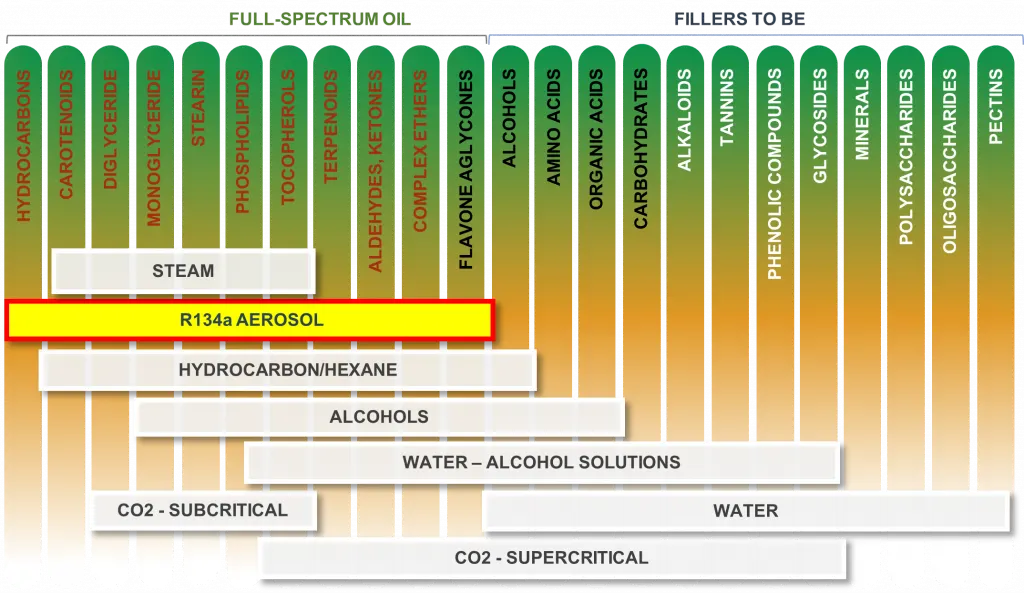
The mold and yeast reside in the organic and amino acid area in the chart and that is why the extraction with ethanol or CO2 will not help mitigating them in the extract. Although using PURE5TM R134a aerosol is the best method of mitigating the mold and yeast. Below are some test data based on 10 different botanicals having various mold concentrations.
We performed the microbial tests according the standard methods of testing molds and yeast and over 12 harmful controlled microbial substances shown in the table below:
| № | MICROORGANISMS | METHOD |
| 1 | Mesophilic anaerobic and facultative anaerobic bacteria | EN ISO 4833:2004 |
| 2 | Moulds | ISO 7954:2002 |
| 3 | Yeast | ISO 7954:2002 |
| 4 | Escherichia coli | ISO 16649-2:2002 |
| 5 | Coliforms | ISO 16649-2:2002 |
| 6 | Other Enterobacteria | ISO 21258-2:2004 |
| 7 | Enterococcus sp. | Slanetz and Bartley |
| 8 | Coagulase-positive staphylococcus | EN ISO 6888-1:2005+A1:2005 |
| 9 | Anaerobic sulfite-reducing bacteria/spores/ | ISO 15213:2003 |
| 10 | Anaerobic sulfite-reducing bacteria/vegetative cells/ | ISO 15213:2003 |
| 11 | Other anaerobic bacteria /spores/ | ISO 15213:2003 |
| 12 | Other anaerobic bacteria/vegetative cells/ | ISO 15213:2003 |
Field Tests
The 12 microorganism activities tested on 16 different plants before and after extraction with PURE5TM R134a Aerosol Extraction machine are clearly showing minimal or no activity after the extraction even if the extraction concentrates the active compounds 10-100 times in various herbs.
Table 1: Mold tests in Sweet Paprika, Black Pepper, Hot Paprika and Pimento:
| Paprika sweet | Black pepper | Paprika hot | Pimento | |||||
| № | Raw material | Extract | Raw material | Extract | Raw material | Extract | Raw material | Extract |
| U | CFU/g | CFU/cm3 | CFU/g | CFU/cm3 | CFU/g | CFU/cm3 | CFU/g | CFU/cm3 |
| 1 | 6×105 | 5×103 | 1.7×106 | 2.5×102 | 6×104 | 1×102 | 1×107 | 5×102 |
| 2 | <101 | <101 | <101 | <101 | <101 | <101 | 2.1×103 | <101 |
| 3 | 2×104 | 1.2×102 | 2×103 | <101 | 1×102 | <101 | <101 | <101 |
| 4 | <102 | <102 | <102 | <102 | <102 | <102 | <102 | <102 |
| 5 | <102 | <102 | <102 | <102 | <102 | <102 | <102 | <102 |
| 6 | <102 | <102 | <102 | <102 | <102 | <102 | <102 | <102 |
| 7 | <102 | <102 | <102 | <102 | <102 | <102 | 2×102 | <102 |
| 8 | 2×102 | <102 | 1.3×103 | <102 | 102 | <102 | 5×103 | <102 |
| 9 | 7×101 | <101 | 8×101 | <101 | 7×101 | <102 | <102 | <101 |
| 10 | <101 | <101 | <101 | <101 | <101 | <101 | 5×101 | <101 |
| 11 | <101 | 1×101 | 1×101 | 1×101 | 2×102 | <101 | <101 | <101 |
| 12 | <101 | <101 | <101 | <101 | <101 | <101 | <101 | <101 |
Table 2: Mold tests in Mint, Marigold, Savory and Chamomile:
| Mint | Marigold | Savory | Chamomile | |||||
| № | Raw material | Extract | Raw material | Extract | Raw material | Extract | Raw material | Extract |
| U | CFU/g | CFU/cm3 | CFU/g | CFU/cm3 | CFU/g | CFU/cm3 | CFU/g | CFU/cm3 |
| 1 | 1×105 | <101 | 1×105 | <101 | 3.4×103 | <101 | 2×105 | <101 |
| 2 | 1.4×102 | <101 | 4×101 | <101 | 2×101 | <101 | 1.2×102 | <101 |
| 3 | <101 | <101 | <101 | <101 | <101 | <101 | <101 | <101 |
| 4 | 1.8×104 | <102 | <102 | <102 | <102 | <102 | 6×104 | <102 |
| 5 | 2×105 | <102 | <102 | <102 | 4×102 | <102 | 2×105 | <102 |
| 6 | 2×105 | <102 | 2×102 | <102 | 8×102 | <102 | 2×105 | <102 |
| 7 | <102 | <102 | <102 | <102 | <102 | <102 | <102 | <102 |
| 8 | <102 | <102 | <102 | <102 | <102 | <102 | <102 | <102 |
| 9 | 1×102 | <101 | 5×103 | <102 | <101 | <101 | <101 | <101 |
| 10 | 4×102 | <101 | 1×103 | <101 | <101 | <101 | <101 | <101 |
| 11 | 2×102 | <101 | 4×104 | <101 | 2×102 | <101 | <101 | <101 |
| 12 | 1×106 | <101 | <101 | <101 | <101 | <101 | 1×106 | <101 |
Table 3: Mold tests in Thyme, Fennel, Basil and Nutmeg:
| Thyme | Fennel | Basil | Nutmeg | |||||
| № | Raw material | Extract | Raw material | Extract | Raw material | Extract | Raw material | Extract |
| U | CFU/g | CFU/cm3 | CFU/g | CFU/cm3 | CFU/g | CFU/cm3 | CFU/g | CFU/cm3 |
| 1 | 4×103 | <101 | 3×103 | <101 | 5×105 | <101 | 2×103 | <101 |
| 2 | 1×102 | <101 | 2×101 | <101 | 2.8×102 | <101 | 3×102 | <101 |
| 3 | <101 | <101 | <101 | <101 | <101 | <101 | <101 | <101 |
| 4 | <102 | <102 | <102 | <102 | 5.4×103 | <102 | <102 | <102 |
| 5 | <102 | <102 | 2.9×102 | <102 | 1×105 | <102 | <102 | <102 |
| 6 | 3×103 | <102 | 1×101 | <102 | 2×105 | <102 | <102 | <102 |
| 7 | <102 | <102 | <102 | <102 | <102 | <102 | <102 | <102 |
| 8 | <102 | <102 | <102 | <102 | 2×104 | <102 | <102 | <102 |
| 9 | 1×103 | <101 | 2×101 | <101 | 6×102 | <101 | <101 | <101 |
| 10 | 8×102 | <101 | 4×102 | <101 | 1×103 | <101 | <101 | <101 |
| 11 | 4×101 | <101 | <101 | <101 | <101 | <101 | <101 | <101 |
| 12 | 2×103 | <101 | 2×103 | <101 | 1×105 | <101 | <101 | <101 |
Table 4: Mold tests in Cinnamon, Clove, Cumin and Cardamom:
| Cinnamon | Clove bud | Cumin | Cardamom | |||||
| № | Raw material | Extract | Raw material | Extract | Raw material | Extract | Raw material | Extract |
| U | CFU/g | CFU/cm3 | CFU/g | CFU/cm3 | CFU/g | CFU/cm3 | CFU/g | CFU/cm3 |
| 1 | 3.1×103 | 8×102 | 1×102 | <101 | 1.25×103 | 1×101 | 2.5×103 | <101 |
| 2 | 9×102 | <101 | <101 | <101 | 3×101 | <101 | 1×101 | <101 |
| 3 | 1×103 | 4.3×102 | <101 | <101 | <101 | <101 | <101 | <101 |
| 4 | <102 | <102 | <102 | <102 | <102 | <102 | <102 | <102 |
| 5 | <102 | <102 | <102 | <102 | <102 | <102 | <102 | <102 |
| 6 | <102 | <102 | <102 | <102 | <102 | <102 | <102 | <102 |
| 7 | <102 | <102 | <102 | <102 | <102 | <102 | <102 | <102 |
| 8 | <102 | <102 | <102 | <102 | <102 | <102 | <102 | <102 |
| 9 | <101 | <101 | <101 | <101 | <101 | <101 | <101 | <101 |
| 10 | <101 | <101 | <101 | <101 | <101 | <101 | <101 | <101 |
| 11 | <101 | <101 | <101 | <101 | <101 | <101 | <101 | <101 |
| 12 | <101 | <101 | <101 | <101 | <101 | <101 | <101 | <101 |
The data collected is clearly showing the PURE5TM Extraction with R134a aerosol mitigates the mold and yeast over 1000 times from the contaminated starting material. This provides another natural and clean way to remediate the contaminated plant while converting in strain specific terpene rich Hash ResinTM extract.
Conclusion
Converting the flower to oil using the correct process is the most effective way to increase the shelf life of a product. The shelf life can be easily 2-5 years if the extract is kept in a cool and dark environment. If the extraction is done with the correct solvent the active ingredients can be preserved as well as the mold and yeast mitigated properly while preserving as well the natural potency of the material.
The extracts made with PURE5TM groundbreaking technology are naturally pure, raw, unaltered by heat, solventless strain specific live resin with preserved health benefits of the original plant. All products are built with naturally extracted ingredients at room temperature and without chemicals. The products are a drop of nature delivered in the most potent natural way.
#
Dr. George Stantchev









