There have been multiple ways to extract cannabis oils on the market and every method created different output. An essential part of keeping the bioavailability of the extract is to repeat the natural consistency of the oil in the plant and maintain neutral PH limiting oxidation, vital low temperature compounds as enzymes, phenolic and flavonoids and amount of the active component loss during the process as terpenes and cannabinoids. In the below article we will discuss the value of those compounds in maintaining the bioavailability of the extract.
Table of Contents
ToggleWhat is an Endocannabinoid system?
The endocannabinoid system is an active and complex cell signaling network. It involves a combination of endocannabinoids, enzymes, and cannabinoid receptors that help regulate several functions in the human body. The discovery of the ECS is relatively new. In the early 1990s, when the chemists isolated the first endocannabinoid in the human brain. Endocannabinoids are similar to the cannabinoids present in the cannabis sativa plant. Therefore they discovered that the human body naturally produces endocannabinoids. The term “endo” refers to “within,” as in within the body.
The endocannabinoid system (ECS) is a biological system in the body that helps regulate and balance key bodily functions. ECS has a potential therapeutic effect that is targeting numerous physiological conditions such as: energy balance, appetite stimulation, blood pressure, pain, embryonic development, nausea and vomiting, control, memory and learning, immune response. In addition, it may target some pathological conditions such as: Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, multiple sclerosis
The endocannabinoids present in various organs and tissues, such as the muscle, brain, and circulating cells become active when they bind with a cannabinoid receptor. The receptors are also located throughout the entire body. The endocannabinoid system is a precise body function regulator. For example, if body temperature is out of the normal range, the ECS regulates it without altering other processes. Once the ECS brings the body back into balance, the enzymes break down the cannabinoids to prevent overcorrecting the problem.
What activates the receptors?
The cannabis plant exerts its pharmaceutical activity primarily by the binding of cannabinoids to two cannabinoid receptors, CB1 and CB2. The activation of CB1 receptors is also achieved by most cannabis terpenes individually, by tetrahydrocannabinol (THC) alone and by THC+terpenes mixtures. The results demonstrate that all terpenes, when tested individually, activate CB1 receptors, at about 10–50% of the activation by THC alone. The combination of some of these terpenes with THC significantly increases the bio-activity of the CB1 receptor, compared to THC alone. In some cases, several times.
Importantly, this amplification is evident at terpene to THC ratios similar to those in the cannabis plant, which reflect very low terpene concentrations. That is why maintaining the natural plant ratios in the extract is essential. For some terpenes, the activation obtained by THC+terpene mixtures is notably greater than the sum of the activations by the individual components, suggesting a synergistic effect. According to recent studies the terpenes have modulatory effects of the cannabinoids and amplify the medical effect in the body by coupling to the CB1 and CB2 receptors. These compositions adjust the effective treatment for various desired medicinal and personal needs.
What is the role of the cannabinoids?
Over 100 phytocannabinoids have been identified, but some of those are artifacts of analysis or are produced in trace quantities. The pharmacology of the more accessible phytocannabinoids will be summarized in Table 1, with emphasis on activities with particular synergistic potential. THC is the most common phytocannabinoid in cannabis drug chemotypes, and is produced in the plant followed by CBD, CBC and CBG.
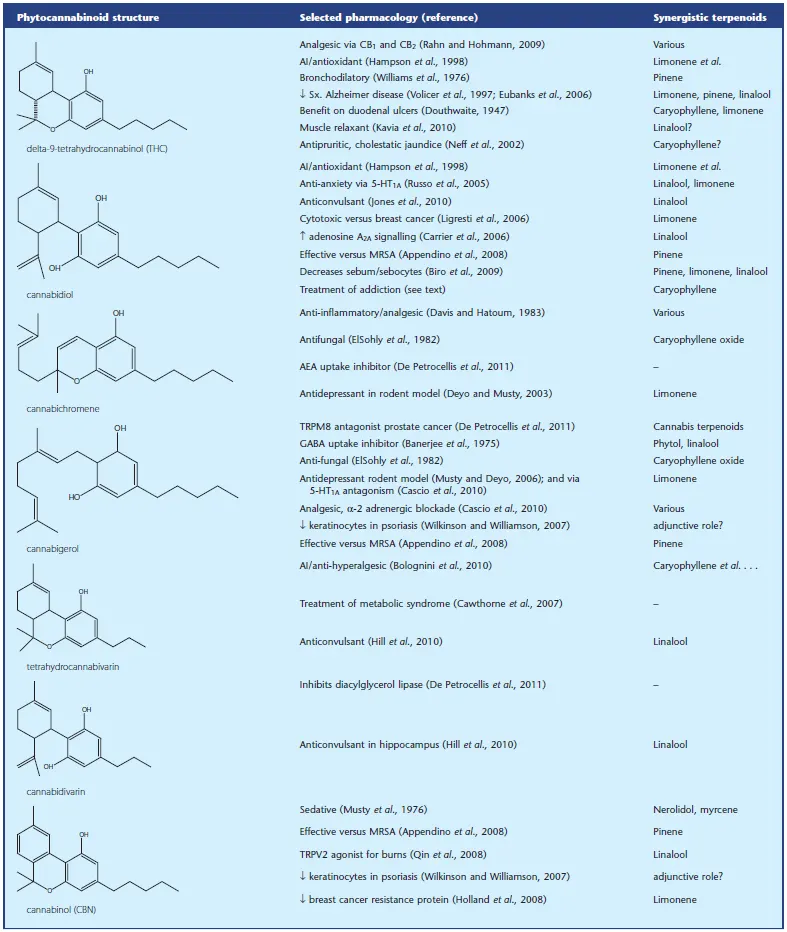
Table 1 – Ethan B Russo, GW Pharmaceuticals, Salisbury, Wiltshire, UK
At Table 1 the most popular 7 type cannabinoids are shown with their adopted pharmacology reference in addressing major physical functions in the body.
What is the role of the terpenes?
Terpenoids are essential oil components, as well conceived as the fifth element, ‘life force’ or spirit and form the largest group of plant chemicals, with 15–20 000 fully characterized properties. Terpenoids, not cannabinoids, are responsible for the aroma of cannabis. Over 200 have been reported in the cannabis plant, but only a few studies have concentrated on their pharmacology. Their yield is less than 1% in most cannabis assays, but they may represent 10% of trichome content due the lack of good extraction techniques.
Monoterpenes usually predominate (limonene, myrcene, pinene), but these volatiles are lost at a rate of about 5% before or during processing due poor knowledge of the extraction techniques. The current extraction technique suffer proper yields within drying, storage and extraction, resulting in a higher relative proportion of sesquiterpenoids (especially caryophyllene), as also often occurs in extracts.
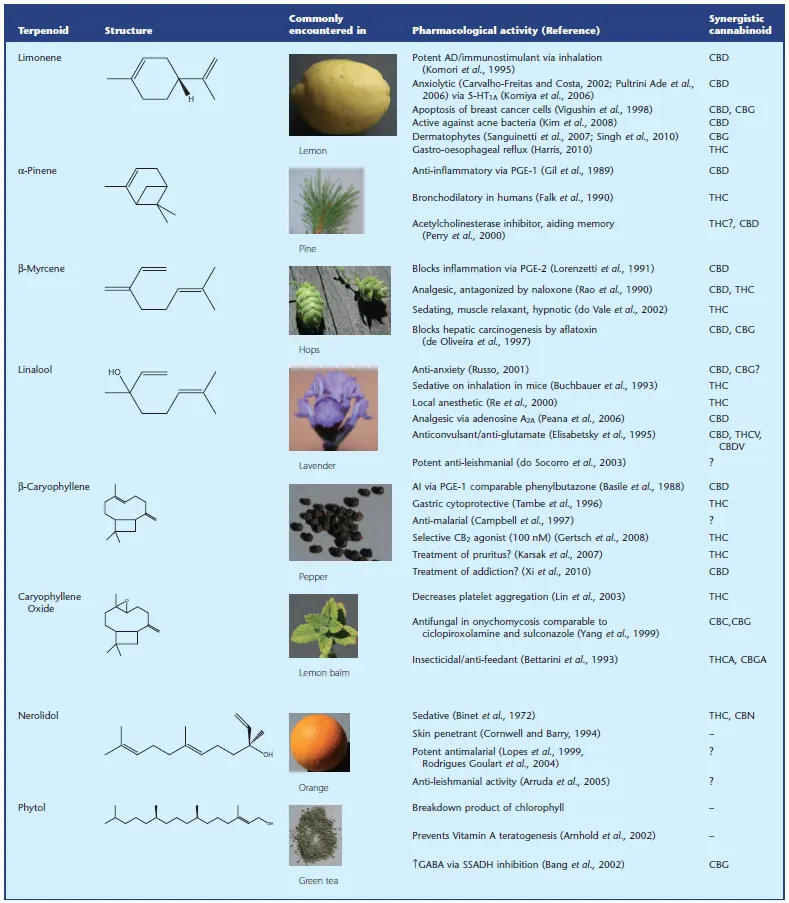
Table 2 – Ethan B Russo, GW Pharmaceuticals, Salisbury, Wiltshire, UK
Table 2 shows the most abundant terpenes in cannabis: Limonene, a-Pinene, b-Myrcene, Linalool, b-Caryophyllene, Nerolidol, Phytol and Caryophyllene Oxide and their pharmacological activity recognized by the pharmacology as well as their affinity to the major cannabinoids as THC, CBD, CBG, THCV, etc.
Considered ensemble, the preceding information supports the concept that selective breeding of cannabis chemotypes rich in phytocannabinoid and terpenoid content offer complementary pharmacological activities that may strengthen and broaden clinical applications and improve the therapeutic index of cannabis extracts containing THC, CBD, or other base phytocannabinoids. This is a firm path into formulating a strain specific medicine science.
Bioavailability increased by terpenes entourage
CB1 activation by Terpenes
Recent studies show that the presence of a number of cannabis terpenes activate the CB1 receptor in degrees ranging between 10 and 50% obtained using similar THC concentrations. A significant dose-dependent CB1 activity was detected for a subset of these terpenes. The role of terpenes in CB1 activation was recently supported by a number of in-vivo studies, demonstrating that the activation effect from selected terpenes is similar to cannabinoid activation rates.
CB1 activation by THC+terpenes mixtures
The results further show that CB1 activation by THC in the presence of β-pinene, borneol, geraniol, limonene, linalool, ocimene, sabinene, and terpineol is significantly increased compared to the activation by THC in the absence of the terpene. This effect is found at terpene/THC weight/weight ratios as close as those found in the cannabis plant.
Terpene modulation of THC interaction with CB1 receptor
Prior studies have suggested various mechanisms for such synergism, including the role of terpenes in modulating THC permeability and absorption, increasing cerebral blood flow, improving THC transport through the blood–brain-barrier and activation of additional signaling pathways complementary to the ECS. The synergism suggests a modulatory effect of the terpene on the interaction between THC and CB1 receptor. This modulation increases the THC-derived CB1 receptor activation several fold (Table 3) and is found at very low terpene concentrations and at terpene/cannabinoids ratios similar to those in the natural cannabis plant.
| Compound | Response normalized to 10 µM THC | Terpene:THC ratio of 1:1 | Terpene:THC ratio of 1:10 |
| THC | 1 | 2.3 | 2.3 |
| α-pinene | 0.29±0.03 | 0.64 | 2.5 |
| β-pinene | 0.29±0.06 | 1.59 | 1.69 |
| β-caryophyllene | N.D2 | N.D2 | 1.98 |
| bisabolol | N.D2 | N.D2 | 2.88 |
| borneol | 0.18±0.03 | 0.59 | 0.49 |
| eucalyptol | 0.41±0.04 | 1.78 | 2.45 |
| geraniol | 0.31±0.08 | 1.21 | 1.4 |
| humulene | N.D2 | N.D2 | 2.37 |
| limonene | 0.23±0.02 | 0.65 | 0.66 |
| linalool | 0.18±0.02 | 1.12 | 1.42 |
| myrcene | 0.26±0.07 | 1.1 | 1.86 |
| nerolidol | N.D2 | N.D2 | 2.01 |
| ocimene | 0.36±0.05 | 0.61 | 0.98 |
| sabinene | 0.1±0.02 | 0.33 | 0.77 |
| terpineol | 0.48±0.05 | 1.12 | 0.82 |
| terpinolene | 0.45±0.02 | 0.82 | 1.92 |
Table 3
THC + Terpenes entourage effect
The term “entourage effect” describes cases wherein co-existence of a compound results in an increased ECS activation by a cannabinoid. Given that cannabis terpenes demonstrate both direct agonism at CB1 receptor and a modulatory effect on THC interaction at CB1 receptor, THC + terpenes effects are beyond the classical definition of entourage.
Recent research shows that terpene-derived CB1 receptor activation provides a major amplification with terpene to THC ratios similar to those in the cannabis plant. The studies demonstrate synergism in selected terpene+THC systems, indicating terpene induced modulation of THC-CB1 receptor interactions. This finding motivates searching for such synergism in other receptor-cannabinoid terpene systems as well.
Terpenes + THC synergism
A possible explanation for the increased CB1 receptor activation by THC in the presence of terpene is an accumulative effect, wherein both the terpene and THC contribute to the receptor activation. Table 4 shows the activation for THC-terpene mixtures to the calculated combined activation of each of the components alone at the corresponding concentration. In the cases of β-pinene and geraniol, the responses of the mixtures are lower than the sum of the contributions, while in the cases of limonene, borneol and sabinene, the responses are notably greater than the sum, suggesting a synergistic effect. To the best of our knowledge, this is the first demonstration of THC- terpene synergism in an in vitro controlled setting. Importantly, synergism here is found at terpene/THC ratios similar to those in the cannabis plant.
| Main effect of condition | |||
| Terpene | df | F | p |
| β-pinene | 13.85 | 1.80 | 0.001 |
| borneol | 1.9 | 23.29 | 0.001 |
| geraniol | 1.7 | 5.68 | 0.02 |
| limonene | 1.6 | 55.84 | 0.001 |
| linalool | 1.65 | 1.84 | 0.18 |
| ocimene | 1.75 | 2.38 | 0.127 |
| sabinene | 1.85 | 11.51 | 0.001 |
| terpineol | 1,100 | 1.71 | 0.193 |
| Main effect of THC concentration level | |||
| Terpene | df | F | p |
| β-pinene | 4.8 | 597.24 | 0.001 |
| borneol | 4.9 | 109.15 | 0.001 |
| geraniol | 4.7 | 214.98 | 0.001 |
| limonene | 4.6 | 398.69 | 0.001 |
| linalool | 4.65 | 460.54 | 0.001 |
| ocimene | 4.75 | 196.91 | 0.001 |
| sabinene | 4.85 | 223.17 | 0.001 |
| terpineol | 4,100 | 141.03 | 0.001 |
| Interaction condition THC concentration levels | |||
| Terpene | df | F | p |
| β-pinene | 4.8 | 3.54 | 0.001 |
| borneol | 4.9 | 2.2 | 0.075 |
| geraniol | 4.7 | 1.56 | 0.194 |
| limonene | 4.6 | 1.66 | 0.174 |
| linalool | 4.65 | 3.1 | 0.022 |
| ocimene | 4.75 | 1.67 | 0.1167 |
| sabinene | 4.85 | 5.37 | 0.001 |
| terpineol | 4,100 | 3.55 | 0.01 |
Table 4
The responses to the co-application of THC with β-pinene and geraniol are larger than the response produced by THC alone and are lower than those expected by summations of their individual responses. However, for borneol, limonene and sabinene the responses to the co-application of THC and a terpene were significantly higher than the response expected from a summation of their individual responses. This shows the high modulation effect of the terpenes that may create a higher response in the body physiology and understanding the proper combination of the selected terpene with the cannabinoid which significantly increases the bioavailability of the selected cannabinoid.
Conclusion
The use of selected terpenes may enable reducing the THC dose in some treatments, and as a result, potentially minimizing the THC-related adverse effects. This would also help in adjusting the treatment to more sensitive populations such as children and elderly. The studies show that the best combination of terpene and cannabinoid mixtures are formed by the natural strain plant profile and those should be maintained through the entire process including extraction for best medical results.
PURE5TM technology resembles the best flower profile in liquid strain specific extract and overcomes the need of infusion of selected terpenes while they have been lost in use of other technologies. We design and manufacture extraction and distillation systems with the objective to create a safe environment for processing and allow anyone to process with consistency and without any chemistry knowledge. Our systems provide high-quality output, speed, and economics for the entire process. The R134a aerosol extracts are obtained at ambient temperature without oxidation, thermal and chemical degradation while preserving the PH normal and full plant profile consistency of the oil.
More info on our equipment and various sizes of the systems you can find on our website:
Pure 5’s Extraction & Remediation Equipment & products.
References
Endocannabinoids: What are they and what do they do? (medicalnewstoday.com)
Endocannabinoid System: A Simple Guide to How It Works (healthline.com)
GPR55 – a putative “type 3” cannabinoid receptor in inflammation (degruyter.com)
The endocannabinoid system: Essential and mysterious – Harvard Health








